- Détection directe fiable des virus de l’herpès simplex de types 1 et 2 ainsi que du virus de la varicelle dans un seul test
- Discrimination des agents pathogènes même si la différenciation clinique est difficile
- Convient pour les prélèvements sur la peau, la bouche ou les parties génitales
Technical data
|
Substrate Test procedure Reagents Controls Test kit format Order number |
Single-stranded DNA probes, length: 15 to 50 nucleotides DNA extraction / PCR (approx. 3 h) / hybridisation (60 min) / fully automated evaluation Ready for use DNA-negative control and other integrated controls 20, 10 or 5 slides each containing 5 test fields, or 8 slides each containing 3 test fields MN 2530 - 2005-1, MN 2530 - 1005-1, MN 2530 - 0505-1, MN 2530 - 0803-1 |
Clinical significance
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) and varicella zoster virus (VZV) are human pathogenic viruses from the
Herpesviridae family that occur worldwide.
HSV-1 and HSV-2, which are closely related, can cause different diseases, with either asymptomatic, mild or severe courses. The most common manifestations are herpes labialis (cold sore) and herpes genitalis, also rarely herpes encephalitis or neonatorum in severe cases. HSV-1 generally leads to cold sores. Genital infections can be caused by both virus types, but mainly by HSV-2. In primary infections the virus penetrates the mucosal cells, multiplies and is released in ulcers or inflammatory skin blisters. Infec- tions persist lifelong, alternating between asymptomatic phases and acute infectious outbreaks. The virus is transmitted by blister fluid or genital secretions at skin or mucosal contact. HSV-1 is also transmitted via the saliva. Infection of the foetus in pregnancy is generally rare. However, if it occurs, it leads to intrauterine death, severe malformations or premature delivery in most cases.
The disease caused by primary infection with VZV is called chickenpox, a highly infectious childhood disease. VZV are trans- mitted by blister fluid, conjunctival fluid or saliva containing the virus. After first manifestation the virus establishes laten- cy in sensory nerve cells and can subsequently reactivate to cause herpes zoster (shingles) as second manifestation. Typical symptoms are painful and/or itching skin exanthema, pains and sensory disorders. However, asymptomatic reactivation has also been described. In severe cases, complications such as pneumonia, hepatitis or post-zoster neuralgia can occur. Since reactivations are associated with a weakened cellular immune response, severe cases occur especially in elderly or immu- nosuppressed persons.
The EUROArray HSV1/2 VZV enables direct detection and differentiation between HSV-1, HSV-2 and VZV based on swab samples from the skin, mouth mucosa or genital mucosa of a patient. It also allows simple and quick discrimination of the pathogens, even if clinical differentiation is difficult. Moreover, it supports the differentiation of herpes infections from other dermatoses with simi- lar symptoms.
Test principle
|
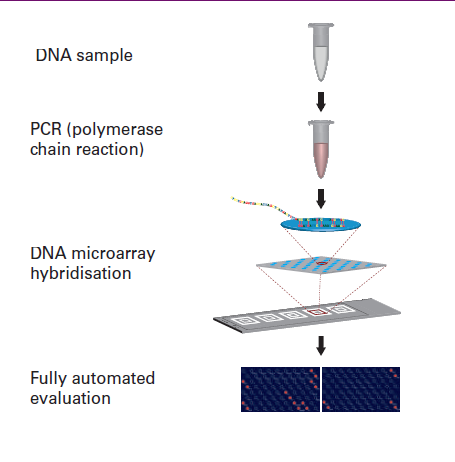
This test system is based on amplification of defined gene sections and sub- sequent detection via a hybridisation reaction with immobilised DNA probes in a microarray system. Isolated DNA from skin, oral or genital swabs of a patient is used as sample material. In the first reaction step, specific DNA sec- tions from the pathogens present in the sample are amplified by polymerase chain reaction (PCR) using a multiplex primer system and, at the same time, labelled with a fluorescent dye. In a second step, the products are detected using an oligonucleotide microarray. The specific binding (hybridisation) of the fluorescence-labelled PCR product to the corresponding oligonucleotide probe is detected using a special microarray scanner. The EUROArrayScan software evaluates all spot signals automatically and deduces the test result. |
 |
Test performance
The PCRs are incubated in the thermocycler and then, using the TITERPLANE technique, incubated on EUROArray slides contain- ing microarray BIOCHIPs. Scanning, evaluation and documentation are performed fully automatically using the EUROArray Scan- ner (incl. EUROArrayScan-Software).
Analytical sensitivity
The lower detection limit (LoD) of this test is 10 DNA copies/reaction for each of the three pathogens. The LOD ist the minimum detection limit. Usually, fewer DNA copies of the pathogenic agent are detected.
Analytical specificity
The specificity of the test system is ensured by the primer and probe design, as well as the PCR and hybridisation conditions given in the test instructions. Using up to 2 million copies of genomic DNA of one of the pathogens to be detected, the test system did not show any cross reactivity with the other pathogens to be detected. Furthermore, no cross reactions were found for high copy numbers of nucleic acids of different other pathogens that occur in or on the human body.
Evalution study
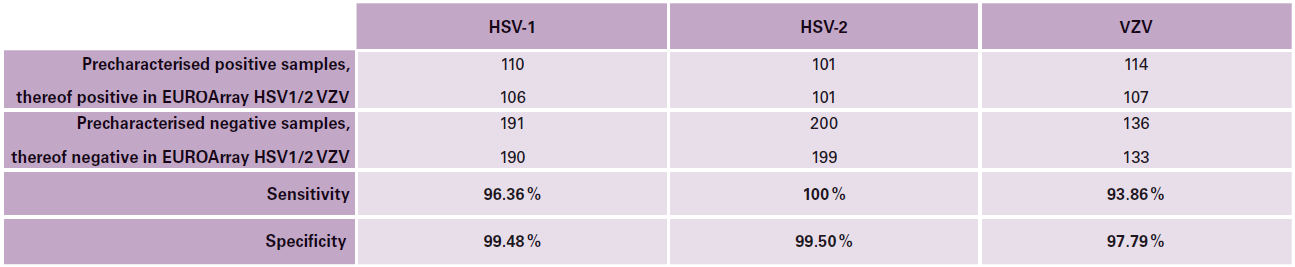
In an evaluation study, swab samples precharacterised by means of PCR-based reference tests were investigated with the EUROArray HSV1/2 VZV: